Biosilicates have been found to have multiple applications in paediatric dentistry. What are they and are they as good as the alternatives?
A literature review. Presented in sunnybank dentist pure dentistry 2019.
SUMMARY
WHAT ARE BIOSILICATES?
- dental cement (including luting agent, liner, base, direct or indirect pulp-capping material) or direct restorative materials
- setting or hardening reaction is based on a hydration reaction between water and hygroscopic inorganic compound(s), including
- calcium silicates
- calcium aluminates
- zinc sulphates
- calcium sulphates
(GMND Agency 2019)
- bioactive materials, owing to the inclusion of tri/dicalcium silicate powder
- Encompass the following:
- MTA-type cements
- tricalcium silicate based cements
- hydraulic calcium silicate cements
- bioactive cements
- Commercially available biosilicates include:
- ProRoot MTA
- Biodentine
- MTA Angelus
- BioRoot RCS
APPLICATIONS OF BIOSILICATES IN PAEDIATRIC DENTISTRY
- indirect pulp capping
- direct pulp capping
- pulpotomy
- partial pulpotomy
- pulpectomy
- root end filling material
- apexogenesis and apexification
- root canal sealer
- repair of perforations
- management of resorption
- horizontal and vertical root fractures
- restorative material
WHAT ARE BIOSILICATES?
There is some ambiguity in the literature as to the classification and nomenclature of biosilicate materials used in dental practice (Ha et al. 2017). For the purpose of this literature review, the term “biosilicates” will be defined as bioactive materials intended for use as a dental cement (including luting agent, liner, base, direct or indirect pulp-capping material) or direct restorative material for which the majority of the setting or hardening reaction is based on a hydration reaction between water and hygroscopic inorganic compound(s), such as calcium silicates, calcium aluminates, zinc sulphates or calcium sulphates (GMND Agency 2019).
Biosilicates are bioactive materials, owing to the inclusion of tricalcium or dicalcium silicate powders (Primus et al. 2019). The term biosilicates may be used to refer to Mineral Trioxide aggregate, tricalcium silicate, di/tricalcium silicate based cements, hydraulic calcium silicate cements and bioactive cements.
History
Calcium silicate Portland cement dates back to Roman times. Adding Pozzolana to concrete helped with setting time even when it was submerged in water. Pozzolana is still the main component of many Portland cements (Prati & Gandolfi 2015). Portland cement was first used in dentistry in the 19th century by Dr Witte. It was later investigated once again by Dr. Torabinejad and Mr White who patented the use of Portland cement in endodontics. The first experimental material was called Mineral Trioxide Aggregate (Lee et al 1993).
Properties of biosilicates:
Expansion and sealing ability: Biosilicates sealing ablity have been tested by different methods such as dye solution, micro-CT scans and polymicrobial tests. Due to the complex 3D porosity distribution micro-CT scans are more accurate than dye solution tests (Primus et al. 2019). The expansion and setting time of these cements can be reduced by presence of proteins in the mixing liquid (Gandolfi et al. 2009). Water sorption increases the expansion and sealing ability. They can expand by 0.2%-6% of the initial volume.
Compressive strength: Condensation pressure and stage of hydration may affect the compressive strength (Nekoofar et al. 2007). Use of acid-etch before restorative procedures may reduce the compressive strength (Watts et al. 2007).
Push out strength: The ability to absorb water and expand increases the microchemical retention of biosilicates on the internal walls and push out strength (Iacono et al. 2010).
Biocompatibility: Some additive materials such as bismuth oxide can affect the biocompatibility of biosilicates (Gandolfi et al. 2009). few biosiliactes have been manufactured for specific use such as direct pulp capping and apexogenesis without any opacifiers to optimize biocompatibility.
Setting time: Setting time of biosilicates can be reduced by presence of Calcium carbonate and proteins and increased in the presence of Naf. (Gandolfi et al. 2009).
Bioactivity and anti-microbial properties: Biosilicates continue to release Ca ions while in contact with moist environment (Gandolfi et al. 2015). Fluoride ion is released in cements containing NaF and can form fluoroapatite (Gandolfi et al. 2010).
Due to alkalizing activity of these cements (Mchugh et al. 2004) and formation of Ca(OH)2
they have antimicrobial action for 15-30 days (Al-Hezaimi et al. 2005).
Setting Reaction
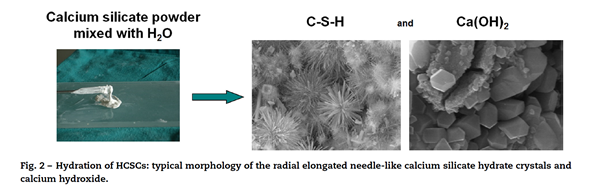
Once the calcium silicate powder and liquid are mixed, at the liquid-solid interface there will be instant dissolution of the ions and calcium release which will form Ca(OH)2 and increase the pH of the environment. The OH ions will attack the silicates and a colloidal gel phase will form (CSH gel). By dissolution and precipitation of the ions in the gel, needle like crystals called calcium silicate crystals and rhomboidal crystals of Ca(OH)2 will form. This will start to harden the gel; the initial hardening happens in about 1-6 hours. It will take a few days for the cement to fully set and all unhydrated calcium silicates to fully hydrate. (Prati & Gandolfi 2015).
Composition
Portland cement used in dentistry is composed of tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite. It may include minor amounts of magnesia, pottasia, soda and sulfates. Various materials are added as opacifiers including Bismuth oxide (MTA), calcium tungstate (MTA-Caps), barium sulphate, Zirconium Oxide (Angelus MTA, MM-MTA, Biodentine) and Tantalum (Bioaggregate) (Camilleri & Gandolfi 2010). Calcium carbonate can be used to reduce the setting time. (Camilleri et al. 2013). Theracal LC is a light cured bioactive cement that contains mostly resin and about 45% hydraulic cement (Arandi & Rabi 2018). It is light cured (fast-setting) and has been claimed to have low solubility and high calcium release (Gandolfi et al. 2011). However, a study by Camilleri et al. showed due to incomplete hydration there was lower calcium ion leach compared to Biodentine and a protype biosilicate cement (Camilleri et al. 2014).
Root-end filling material
A root end filling material may need to be placed in an immature nonvital tooth before obturation of the canal. Materials such as amalgam, resin composites, gutta-percha, IRM and biosilicates can be used.
D’Anto et al. study reported MTA can promote tissue healing (D’Antò et et al. 2010). MTA is able to form cementum and bone in the periapical area in animal studies (Katsamakis et al. 2013). A prospective non-randomized study reported biosilicates were more effective than Resin composites after 5 years of follow up (Von Arx et al. 2014). Saatchi et al. reported that one week after placing amalgam at the open root apex mercury blood levels can increase (Saatchi et al. 2013). Custom fitted gutta-percha can also be placed at the open apex. To perform this technique the coronal segment needs to be prepared wider than the apical section which will increase chances of fracture (Rafter et al. 2005). Gutta percha can be back filled and smoothened at the root by surgery. Use of MTA compared to this method showed better healing (96%) compared to use of back filled Gutta Percha 52% and smoothening it with surgery (Christiansen et al. 2009). Although study by Saunders concluded microsurgical technique in combination with MTA had high clinical success rate (Saunders et al. 2008) other studies by Von Arx et al. and Song and Kim showed no significant difference between use of Super EBA and MTA after apical surgery (Von Arx et a.l 2012 & Song and Kim 2012).
Indirect pulp capping
Indirect pulp treatment is a procedure performed in teeth with a deep carious lesion approximating the pulp but without signs or symptoms of pulp degeneration. (AAPD 2008)
IPC has a success rate of about 90%. According to Coll et al. success rate usually does not depend on the medicament or technique (Coll 2008). Indirect pulp capping procedure success is highly dependent on a well-sealed coronal restoration, as study by Besic reported, liner placed in a deep carious lesion is not to stop the carious process, as that stops after placing a well-sealed restoration on the tooth but to eliminate any bacteria present in the deep carious lesion (Besic 1943). Study by Dalpian et al. also reported no difference in dentine microhardness of primary teeth undergoing partial caries removal when using dycal or not using anything (Dalpian et al. 2012).
Different materials such as Ca(OH)2,GIC and biosilicates have been used as medicaments for IPC.
Garrocho-Rangel et al. reported no significant difference between using Ca(OH)2 and biosilicate cement in IPC success rate in primary teeth (Garrocho et al. 2017).
Petrou et al. compared the use of nonabsorbable MTA and Portland cement with Ca(OH)2 and reported IPC with MTA induced a harder dentin and presence of less bacteria in 6 month compared to Ca(OH)2. They concluded although success rate for all medicaments used were the same, the use of non-resorbing MTA cements as one visit IPC is more favorable to use of Ca(OH)2 (Petrou et al. 2014). Some of the disadvantages of Ca(OH)2 is that it is absorbed more over time compared to MTA (Fridland et al. 2005). Another drawback is disintegration and formation of tunnel defects in the newly formed dentin by Ca(OH)2 (Olsson et al. 2006). Study by Hashem et al. compared biodentine with GIC and reported the clinical efficacy were the same for both materials in patients with reversible pulpitis (Hashem et al. 2015).
APPLICATIONS OF BIOSILICATES
In this review, some of the current applications of biosilicate materials in paediatric dentistry will be discussed. These include procedures completed in both primary and young permanent teeth. The applications that will be investigated further are direct and indirect pulp capping, pulpotomy and partial pulpotomy. Applications in young permanent teeth include use as a root end filling material, a root canal sealer, in apexogenesis and apexification, for repair of perforations, in the management of resorption, horizontal and vertical root fractures and as a restorative material.
Partial Pulpotomy
Cvek reported one of the landmark studies on partial pulpotomy technique in 1978. The technique involves the surgical removal of only the superficial layers of the exposed or inflamed pulp to reach the deeper healthy pulp tissue. The pulpal wound is then dressed after pulpal bleeding has been controlled through irrigation with a bacteriocidal solution such as sodium hypochlorite or chlorhexidine (Mejare & Cvek 1993). Partial pulpotomy is carried out for young permanent teeth that suffer carious pulp exposure (AAPD 2015). Permanent teeth that suffer pulp exposure secondary to traumatic crown fractures are usually treated with the Cvek pulpotomy technique (AAPD 2015). This involves removal of one to three millimeters of pulp tissue adjacent to the traumatic pulp exposure. The pulp is then covered with a dressing material such as calcium hydroxide (Cvek 1978).
Calcium hydroxide had long been the gold standard pulp dressing in the partial pulpotomy technique. More recently biosilicate materials such as Mineral Trioxide Aggregate have been advocated for use as a pulp dressing agent (AAPD 2015, Özgür et al. 2016). Ca(OH)2 has been demonstrated to have good long-term success rates (Mejàre & Cvek 1993). However, there is evidence of more predictable dentine bridge formation and better pulpal response with the use of MTA (AAPD 2015, Chako & Kurikose 2016). When treating anterior permanent teeth, using white MTA rather than gray MTA helps to lower the chances of intrinsic tooth discoloration (Bakland 2019).
A review by Bimstein & Rotstein (2016) presented an update on Cvek pulpotomy technique. They reported success rates for treatment with MTA and Ca(OH) 2 concluded that there was no statistically significant difference in the success rates of the technique when treated with either material. Instead, they reported that the key factor in success was the quality of the seal to prevent microbial invasion to the remaining pulp.
Pulpotomy
Another frequently used application of biosilicates is in pulpotomy treatment. Pulpotomy is a form of vital pulp therapy performed on primary teeth with extensive caries in the absence of signs or symptoms of radicular pathology (AAPD 2014). This usually occurs in the case of carious or mechanical pulp exposure encountered during caries removal or following traumatic pulp exposure.
The aim of pulpotomy treatment is to retain the tooth in function to preserve the integrity of the alveolus. This is achieved by preservation of the radicular pulp tissue and ensuring the patient remains free of pain, swelling and infection (Fuks 2008). The rationale behind the pulpotomy procedure hinges on the ability for the radicular pulp tissue to retain its capacity to heal or remain healthy following surgical amputation of the effected coronal pulp tissue (Fuks 2002). Success of treatment however depends largely on establishing a proper diagnosis. A diagnosis of normal pulp or reversible pulpitis should be confirmed via clinical and radiographic findings prior to considering treatment with pulpotomy.
With regards to medicament choice following coronal pulp amputation, the dressing material should possess a number of qualities as follows:
- harmless to the radicular pulp and surrounding tissues
- bactericidal effect
- promote tissue regeneration and healing of the radicular pulp
- possess good sealant properties
- support regeneration of the dentine-pulp complex
- avoid any interference with the physiologic root resorptive process
(Fuks 2002).
A number of materials have been investigated for use as a pulpotomy medication, including biosilicate materials more recently. In order to assess the efficacy of biosilicates in this application of paediatric dentistry, this review will investigate some of the alternative material choices applied to pulpotomy procedures.
Pulpotomy technique can be classified by the treatment objectives of devitalisation, preservation or regeneration (Ranly 1994). It is worth noting that certain medications can be applied to multiple treatment objectives. Devitalisation pulpotomy originally involved the complete mummification of the medicated pulp tissue, theoretically leaving the radicular pulp sterile and vital. This technique is exemplified with the use of Formocresol (FC) as the medicament. The use of FC was introduced as a multiple visit technique by Sweet in 1930 and aimed to completely mummify the tissue. A five–minute formocresol technique was tested by Redig in 1968, which went on to become the mainstay of FC pulpotomies in the practice of paediatric dentistry. Electrosurgical technique is another example of devitalisation pulpotomy. The benefits of electrosurgical pulpotomy include its non-pharmacological nature, ease of use and favourable success rates (Dean et al 2002).
Preservation pulpotomy aims to minimise pulpal irritation and conserve as much of the radicular pulp as possible, without attempting to induce reparative dentine formation. Examples of medicaments used in the preservation pulpotomy technique include glutaraldehyde and Ferric Sulphate (Ranly 1994). Ferric sulphate works as a coagulative and haemostatic agent. This is achieved through formation of a metal-protein complex on contact with blood in the radicular pulp tissue (Epstein & Maibach 1964).
Finally, regeneration pulpotomy technique involves the stimulation of dentine bridge formation (Ranly 1994). Various medicaments can be used in regeneration pulpotomy technique. These include Calcium Hydroxide (Ca(OH)2) and several types of biosilicate materials, such as Mineral Trioxide Aggregate (MTA) and Biodentine. Calcium hydroxide was one of the first pulpotomy medicaments that demonstrated the ability to induce regeneration of dentine (Zander 1939). However, several issues have since been documented in Ca(OH)2 pulpotomies including internal resorption (Sonmez 2008) and higher rates of failure (Huth et al. 2012).
In 2001, Eidelman et al. presented a preliminary report comparing the use of Mineral Trioxide Aggregate to FC as a pulp-dressing agent in the pulpotomy of primary molars .The report noted promising clinical and radiographic findings after use of MTA as a pulp dressing material and suggested it may replace FC in the future. The authors did however acknowledge the limitations of the study including the small sample size (17 teeth in the MTA experimental group, 15 teeth in the FC control group) and short follow up time. On the basis of the sample size, it is not possible to draw definitive conclusions about MTA as a pulpotomy medicament from this report (Eidelman et al. 2001).
A systematic review and meta-analysis by Stringhini et al (2015) compared MTA, formocresol, ferric sulfate and electrosurgery pulpotomy. It found that MTA significantly improved success of pulpotomy, deeming MTA a better pulpotomy medicament that FC. It also found FS and electrosurgery pulpotomy had similar success rates to FC.
Coll et al. conducted a systematic review and meta-analysis on primary tooth vital pulp therapy. They compared the clinical and radiographic performance and success rates of several pulpotomy medications. Some of the conclusions are summarised below:
- MTA and FC had the highest pulpotomy success rates at 24 months follow up, at 89.6% and 85.6% respectively. There is high quality evidence supporting the conclusion that the success rates of MTA and FC were not significantly different
- 24 month success rates for MTA, FC and FS were all significantly higher than Ca(OH)2. Therefore, the authors do not recommend the use of Calcium hydroxide as a pulpotomy medicament (Coll et al. 2017).
A recent Cochrane review by Smaïl-Faugeron et al. (2018) investigated pulpotomy medicaments in the treatment of primary teeth with extensive caries. The following three comparisons were found to have moderate-quality evidence:
- MTA reduced the clinical and radiographic failure rate of pulpotomy in comparison to formocresol. This difference was statistically significant at 12 months for clinical failure and at six, 12 and 24 months for radiographic failure
- MTA reduced the failure rate in comparison to calcium hydroxide. The difference was statistically significant at 12 and 24 months for clinical failure and at six, 12 and 24 months for radiographic failure
- when comparing formocresol to calcium hydroxide, formocresol was found to have better clinical and radiographic success rates at a statistically significant level at six and 12 months
Smaïl-Faugeron et al. went on to conclude that MTA was likely the best medicament to use in pulpotomy treatment of primary teeth. The authors also stated that Biodentine, enamel matrix derivatives or laser treatment are good alternatives where MTA is not accessible. It must also be taken into consideration that all of the trials included in this review were assessed to have an unclear or high level of bias.
Direct Pulp Capping
Direct pulp capping is applying a medicament on the exposed pulp to try to preserve pulp vitality and to induce formation of a new dentine bridge to protect the dental-pulpal tissue. DPC can be performed on a tooth with a healthy pulp that has been exposed from a traumatic injury or by iatrogenic means (Fuks 2008). Pulp capping material should be bactericidal or bacteriostatic ,biocompatible, radiopaque, maintain vitality of tooth, adhere to dentine. Gaining a dentinal bridge is the main purpose of DPC (Stanley 1989). Success of DPC depends on patients age, size of exposure, remaining tooth structure, and achieving hemostasis (Aeinechi et al. 2003). Biosilicates, Zinc Oxide Eugenol, Glass Ionomer and resin modified glass ionomer, adhesive restorations and calcium hydroxide have been used for DPC. Biodentine has showed superior physical and biological properties compared to MTA (Rajasekharan et al. 2018).
Ca(OH)2: Different variables are studied when comparing biosilicates with Ca(OH)2. A systematic review and meta-analysis by Paula et al. reported Intense inflammatory response (21.4%-%100) for Ca(OH)2 and (0%-80%) for MTA. Dentin bridge formation varied between 0%-80% for Ca(OH)2 and 33.3%-100% for MTA. Clinical success rate for Ca(OH)2 was 69.5%-96% and 80.3%-100% for MTA (Paula et al. 2018).Microorganisms were absent in 95% of cases when treated with Ca(OH)2 and 100% when MTA was used (Acorinte et al. 2008). A preliminary report on human teeth by Aeinechi et al. reported a thicker dentinal bridge and less pulpal inflammation with MTA compared to Ca(OH)2 (Aeinechi et al. 2003). Study by Nair et al. suggested MTA was clinically easier to use as a direct pulp-capping agent and resulted in less pulpal inflammation and more predictable hard tissue barrier formation than Dycal (Nair et al. 2008). A randomized clinical trial by Hilton et al. also confirmed superior performance of MTA as a DPC material compared to Ca(OH)2(Hilton et al. 2013). Both Ca(OH)2 and MTA need to be sealed. This is to prevent leakage when Ca(OH)2 is used and to protect MTA before placing a restoration during slow setting time. MTA has shown to have better sealing ability than Ca(OH)2 (Luketic et al. 2008). Study by Cox et al. concluded that there was multiple tunnel defects in the formed dentinal bridge by Ca(OH)2, it washes out and disintegrates after 6 months (cox et al. 1996).
ZOE: Review of literature by Glass et al, reported use of ZOE as DPC can cause chronic inflammation and does not produce a calcific barrier. Most of the time the result is necrosis of the tooth (Glass & Zander 1949).
Composite resin: Although Kitasko et al. reported no permanent irritation to the pulp when direct restorative material were used in contact with pulp of monkeys teeth (Kitasako et al. 1999) and Schuurs et al. suggested use of composites and adhesive systems for DPC (Schuurs et al. 2000) , other studies by Horsted et al. have shown partial dentin bridge formation when composite restorations are used for DPC(Hörsted-Bindslev et al. 2003 and Nowicka et al. 2016). Elias et al. reported dentin bridge may only form 90 days after use of adhesive restoration material for DPC (Elias et al. 2007). This may increase susceptibility of the pulp to inflammation by bacteria due microleakage. Uncompleted polymerization of resin due to presence of oxygen and moisture at exposure site and diffusion into the dentinal tubules may cause cytotoxic effects on pulp (Suzuki et al. 2005). This method has been controversial, and most studies have shown poor results in the success rate (Crane et al. 2016). According to a systematic review by Paula et al. lowest success rate was when dental adhesive systems were used (Paula et al. 2018).
IRM: Chang et al. reported Bioaggregate and micromega MTA promoted mineralization and mRNA expression for osteogenic/odontogenic markers with superior results compared to IRM (Chang et al. 2014).
Hydroxyapatite: A few animal studies have shown absence of dentin bridge formation (Danesh et al. 2012) ,dystrophic calcification (Jaber et al. 1991) and pulp necrosis in presence of hydroxyapatite (Hebling et al. 1999). Not enough long-term studies have been done on this material to be able to compare it with biosilicates.
Enamel Morphogenetic proteins: EMP have been proposed as a promising material for DPC in some animal studies. Paula et al, concluded in her systematic review that there was inflammatory responses in 50%-100% of the cases and no bridge formation when EMD was used a DPC material (Paula et al. 2018).
Laser: Laser has also been reported to have high success rate of about 88% when used for DPC. (Deng et al 2016). It is not possible to conclude that the success rate is completely due to laser use as it is dependent on type of material (Ca(OH)2 or MTA) used after laser application.
Other materials that have been proposed for DPC include calcium Phosphate cement, MTY A1-Ca, bonesialoprotien, Simvastatin, enzymes, Propolis, novel endodontic cement, Emdogain, Odontogenic ameloblast associated protein, castor bean oil. There has not been many studies to compare clinical applications of these material with calcium silicate based cements.
Apexogenesis & Apexification
Apexogenesis (root formation) is a term used to describe the continued physiologic development and formation of the root’s apex and can be achieved by IDP, DPC and partial pulpotomy. (AAPD 2008)
Apexification (root end closure) is a method of inducing root end closure of an immature nonvital permanent tooth by removing the coronal and nonvital radicular tissue and placing a biocompatible material in the canal. In instances when complete closure cannot be accomplished a barrier can be placed at the root end to allow. (AAPD 2008)
Biosilicates such as MTA and Biodentine, Ca(OH)2 can be used for apexification and apexogenesis.
Disadvantages of using calcium hydroxide compared to biosilicates is, need for multiple visits and more treatment time (Abott 1998), induced changes to dentine and consequently increased fracture risk. It has been proposed by Cvek that immature teeth are weakened by filling of the root canals with calcium hydroxide dressing and gutta‐percha (Cvek 1992). By using Biosilicates and forming a root barrier in one session the coronal permanent restoration can also be placed sooner which will reduces chances of coronal leakage. Study done by Parirokh and Torabinejad also showed reduced root fractures compared to Ca(OH)2 (Parirokh & Torabinejad 2010b) which was similar to the findings by Bonte et al. In their study 4 out of 15 teeth exhibited coronal or radicular root fracture when Ca(OH)2 was sued. They have also reported better and faster results of apical hard tissue forming when MTA was sued (Bonte et al. 2015). However they both performed the same as producing an apical seal after 6-12 months which was confirmed in a systematic review by Chala et al. (Chala et al. 2011).
Perforation
Endodontic perforation is a mechanical or pathological communication between the root canal system and the periradicular tissues or the oral cavity (AAE 2019). Mechanical perforations occur due to iatrogenic causes such as misalignment of endodontic burs when attempting to negotiate complex root canal anatomy or locate calcified root canal orifices (Fuss & Trope 1996). The prognosis of the tooth following perforation depends on location of the perforation, duration that the area is contaminated and accessibility to seal the defect (Sinai 1977). Some of the materials advocated for use in repair of perforations includes MTA, amalgam, decalcified freeze-dried bone, gutta percha, Ca(OH)2, calcium sulphate and hydroxyapatite (Tsesis & Fuss 2006) Calcium hydroxide based materials are unsuitable for use in repair of crestal and furcation perforations as they induce an initial inflammatory response in the tissues. This could lead to breadown of the supporting periodontal tissues and result in pocket formation (Himel et al. 1985).
Overall success rate of about 81% of perforation repair using MTA was reported in a systematic review by Siew et al (Siew et al. 2015). Study by Main et al. demonstrated teeth with existing lesions showed resolution of the lesion, and teeth without preoperative lesions continued to demonstrate absence of lesion formation at the follow-up visit (Main et al. 2004). Compared to amalgam when used straight after perforation MTA showed better success rate (Ford et al. 1995), The biocompatibility of MTA has been found to be equal or superior to amalgam and IRM (Schmitt et al. 2001).
Formation of fibrous tissue when using resin composite, IRM, Zinc ethoxybenzoic acid cement is common. There is usually some degree of chronic inflammation in the periodontal tissue adjacent to these materials. Sealing ability of the mentioned material is also compromised with moisture. Zinc oxide eugenol caused inflammation when in contact with tissue and was not successful for treatment of perforations (Bramante & Berbert 1987).None of the old material can ensure sealing of the perforation as they do not have osteogenic, cementogenic and antibacterial properties (Holland et al. 2007).
Super Ethoxy Benzoic Acid (Super EBA) is a Alumina-reinforced zinc oxide-eugenol cement. Super EBA has very good adaptation to dentinal walls and has high adhesiveness to dentine. It is also biocompatible with the periapical tissue (Oynick & Oynick 1985). Super EBA used with MTA can produce a faster seal than MTA alone. At 24 hours EBA showed less microleakage compared to silver GIC and Amalgam (Weldon et al. 2002)
Intermediate Restorative Material (IRM) is a reinforced zinc oxide eugenol cement. Biosilicates have much less leakage compared to IRM (Lee et al. 1993).
Guttapercha was introduced by Bowman in 1867. It showed a high failure rate when used as perforation repair material (Benenati et al. 1986).
Resorption
Biosilicates have been used for management of both internal and external root resorption. Used in invasive cervical root resorption, internal root resorption as well as inflammatory root resorption.
Some animal studies have reported no significant difference between Ca(OH)2 and biosilicate as root filling material after re-implantation (Esteves et al. 2015 and Von Arx et al. 2007). However other animal studies have reported biosilicates such as MTA and CEM as root filling material can have successful treatment of inflammatory resorption following unsuccessful treatment with Ca(OH)2. (Aggarwal & Singla 2010 and Sahrifi et al. 2014)
Vertical root fracture
Vertical root fracture has as a poor prognosis, but some studies have reported successful clinical outcome by using MTA (Floratos & Kratchman 2012, Hadrossek & Dammashcke 2014). These studies are only case reports.
Root canal sealer
Conventional endodontic treatment is indicated for apexified permanent teeth with irreversible pulpitis or pulpal necrosis (AAPD 2015). The aim of root canal treatment is to disinfect and subsequently to fill the root canal system to prevent bacterial and fluid entry. Root canal sealers are traditionally used to create an interface between the obturation material and the dentine walls to ensure adequate sealing of the root canal space (Giacomino et al. 2019). The ideal properties of a root canal sealer include the following:
- slow setting
- insoluble in tissue fluids
- well tolerated and non-irritating to the periradicular tissues
- soluble in common solvents if it is necessary to removed the root canal filling
(Grossman 1982)
Root canal sealers can be classified by their chemical composition as calcium-hydroxide containing, zinc oxide eugenol (ZOE) based, epoxy-resin based and calcium silicate based (Seo et al. 2019). These calcium silicate based sealers fall under the classification of biosilicate materials, which have added benefits due to their bioactivity (Giacomino et al. 2019). A study by Giacomino et al. investigated the biocompatibility and osteogenic capacity of biosilicate sealers (ProRoot ES and EndoSequence BC Sealer) in comparison to a conventional ZOE (Roth) and epoxy-resin based sealer (AH Plus). They found that both biosilicate sealers tested significantly enhanced osteoblastic differentiation in comparison to the ZOE and resin based sealers. This is a significant finding given that osseous healing following endodontic treatment is largely dependent on the differentiation and activity of osteoblasts (Giacomino et al. 2019).
Restorative material
Due to low compressive strength, biosilicates are generally not recommended as permanent restorative materials (Parirokh and Torabinejad 2010a). Biodentine has been recommended as a temporary restorative material. Abrasion of the restoration was major failure of biodentine after 6 months (Koubi et al. 2013).
Horizontal root fracture
The survival rate of a horizontally root-fractured tooth is reported to be relatively high (83%) for up to 10 years of observation (Cvek et al. 2008). Biosilicates have been used for treatment of horizontal root fractures successfully by root filling the coronal or apical fragment (Cvek et al. 2008 and Parirokh & Torabinejad 2010b). One of the major drawbacks of this finding is that the majority of the reported literature is based on case reports alone.
CONCLUSION
Biosilicate materials have multiple applications in paediatric dentistry. A range of materials fall under the classification of biosilicates, most of which vary in their composition and therefore in their physical properties, handling characteristics and applications. A majority of the literature focuses on MTA materials alone. With many new generation biosilicate materials on the market, further research is required to investigate the properties and applications of biosilicate materials in comparison to their alternatives in the practice of paediatric dentistry.
REFERENCES
Abbott, P. V. (1998). Apexification with calcium hydroxide–when should the dressing be changed? The case for regular dressing changes. Aust Endod J, 24(1), 27-32.
Accorinte Mde, L., Holland, R., Reis, A., Bortoluzzi, M. C., Murata, S. S., Dezan, E., Jr., . . . Alessandro, L. D. (2008). Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod, 34(1), 1-6. doi:10.1016/j.joen.2007.09.012
Aeinehchi, M., Eslami, B., Ghanbariha, M., & Saffar, A. S. (2003). Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J, 36(3), 225-231.
Aggarwal, V., & Singla, M. (2010). Management of inflammatory root resorption using MTA obturation – a four year follow up. Br Dent J, 208(7), 287-289. doi:10.1038/sj.bdj.2010.293
Al-Hezaimi, K., Al-Hamdan, K., Naghshbandi, J., Oglesby, S., Simon, J. H., & Rotstein, I. (2005). Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. Journal of endodontics, 31(9), 684-686.
American Academy of Pediatric Dentistry. Guideline on pulp therapy for primary and immature permanent teeth. Pediatr Dent 2015;37:244–52.
Arandi, N. Z., & Rabi, T. (2018). TheraCal LC: From Biochemical and Bioactive Properties to Clinical Applications. International journal of dentistry, 2018.
Bakland LK. New endodontic procedures using mineral trioxide aggregate (MTA) for teeth with traumatic injuries. In: Andreasen JO, Andreasen FM, Andersson L, eds. Textbook and Color Atlas of Traumatic Injuries to the Teeth. 5th ed. Ames, Iowa: Blackwell Munksgaard; 2019: 659-63.
Benenati, F. W., Roane, J. B., Biggs, J. T., & Simon, J. H. (1986). Recall evaluation of iatrogenic root perforations repaired with amalgam and gutta-percha. J Endod, 12(4), 161-166.
Besic, F. (1943). The fate of bacteria sealed in dental cavities. Journal of Dental Research, 22(5), 349-354.
Bimstein E & Rotstein I. Cvek Pulpotomy – revisited. Dent Traumatol 2016; 32: 438–442.
Bonte, E., Beslot, A., Boukpessi, T., & Lasfargues, J. J. (2015). MTA versus Ca(OH)2 in apexification of non-vital immature permanent teeth: a randomized clinical trial comparison. Clin Oral Investig, 19(6), 1381-1388. doi:10.1007/s00784-014-1348-5
Bramante, C. M., & Berbert, A. (1987). Root perforations dressed with calcium hydroxide or zinc oxide and eugenol. J Endod, 13(8), 392-395. doi:10.1016/s0099-2399(87)80200-5
Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J 2008;41:408–17
Camilleri, J., & Gandolfi, M. (2010). Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. International endodontic journal, 43(1), 21-30.
Camilleri, J., Laurent, P., & About, I. (2014). Hydration of biodentine, Theracal LC, and a prototype tricalcium silicate–based dentin replacement material after pulp capping in entire tooth cultures. Journal of endodontics, 40(11), 1846-1854.
Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pitt Ford TR. The constitution of mineral trioxide aggregate. Dent Mater 2005; 21: 297–303
Camilleri, J., Sorrentino, F., & Damidot, D. (2013). Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dental Materials, 29(5), 580-593.
Chacko V & Kurikose S. Human pulpal response to mineral trioxide aggregate (MTA): a histologic study. J Clin Pediatr Dent 2006; 30(3), 203-209.
Chala, S., Abouqal, R., & Rida, S. (2011). Apexification of immature teeth with calcium hydroxide or mineral trioxide aggregate: systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 112(4), e36-42. doi:10.1016/j.tripleo.2011.03.047
Chang, S. W., Lee, S. Y., Kum, K. Y., & Kim, E. C. (2014). Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endod, 40(1), 113-118. doi:10.1016/j.joen.2013.09.036
Chaniotis, A. (2014). The use of MTA/blood mixture to induce hard tissue healing in a root fractured maxillary central incisor. Case report and treatment considerations. Int Endod J, 47(10), 989-999. doi:10.1111/iej.12237
Choi, Y., Hong, S.-O., Lee, S.-R., Min, K.-S., & Park, S.-J. (2014). Healing after horizontal root fractures: 3 cases with 2-year follow-up. Restorative dentistry & endodontics, 39(2), 126-131.
Christiansen, R., Kirkevang, L. L., Hørsted‐Bindslev, P., & Wenzel, A. (2009). Randomized clinical trial of root‐end resection followed by root‐end filling with mineral trioxide aggregate or smoothing of the orthograde gutta‐percha root filling–1‐year follow‐up. International endodontic journal, 42(2), 105-114.
Coll, J. A. (2008). Indirect pulp capping and primary teeth: is the primary tooth pulpotomy out of date? Pediatr Dent, 30(3), 230-236.
Cox, C. F., Subay, R. K., Ostro, E., Suzuki, S., & Suzuki, S. H. (1996). Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent, 21(1), 4-11.
Crane, L. E. (2006). Hard tissue barrier formation after pulp capping? Evidence-based dentistry, 7(4), 95.
Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture 1978; 4(8): 232-237
Cvek, M., Tsilingaridis, G., & Andreasen, J. O. (2008). Survival of 534 incisors after intra‐alveolar root fracture in patients aged 7–17 years. Dental Traumatology, 24(4), 379-387.
D’Antò, V., Di Caprio, M. P., Ametrano, G., Simeone, M., Rengo, S., & Spagnuolo, G. (2010). Effect of mineral trioxide aggregate on mesenchymal stem cells. Journal of endodontics, 36(11), 1839-1843.
Dalpian, D. M., Casagrande, L., Franzon, R., Dutra, G. M., & de Araujo, F. B. (2012). Dentin microhardness of primary teeth undergoing partial carious removal. J Clin Pediatr Dent, 36(4), 363-367.
Damle, S., Bhattal, H., Damle, D., Dhindsa, A., Loomba, A., & Singla, S. (2016). Clinical and radiographic assessment of mineral trioxide aggregate and calcium hydroxide as apexification agents in traumatized young permanent anterior teeth: A comparative study. Dental research journal, 13(3), 284.
Danesh, F., Vahid, A., Jahanbani, J., Mashhadiabbas, F., & Arman, E. (2012). Effect of white mineral trioxide aggregate compared with biomimetic carbonated apatite on dentine bridge formation and inflammatory response in a dental pulp model. International endodontic journal, 45(1), 26-34.
Dean JA, Mack RB, Fulkerson BT, Sanders BJ. Comparison of electrosurgical and formocresol pulpotomy procedures on children. Int J Paediatr Dent 2002; 12:177– 182
Demarco, F. F., Tarquinio, S. B. C., Jaeger, M. M. M., de Araújo, V. C., & Matson, E. (2001). Pulp response and cytotoxicity evaluation of 2 dentin bonding agents. Quintessence Int, 32(3).
Deng, Y., Zhu, X., Zheng, D., Yan, P., & Jiang, H. (2016). Laser use in direct pulp capping: A meta-analysis. J Am Dent Assoc, 147(12), 935-942. doi:10.1016/j.adaj.2016.07.011
Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: A preliminary report. Pediatr Dent 2001;23:15-18
Elias, R. V., Demarco, F. F., Tarquinio, S. B., & Piva, E. (2007). Pulp responses to the application of a self-etching adhesive in human pulps after controlling bleeding with sodium hypochlorite. Quintessence Int, 38(2).
Epstein E, Maibach H. Monsel’s solution: history, chemistry and efficacy. Arch Dermatol 1964; 90: 226-228
Esteves, J. C., Marão, H. F., dos Santos Silva, P. I., Poi, W. R., Panzarini, S. R., Aranega, A. M., . . . Sonoda, C. K. (2015). Delayed tooth replantation following root canal filling with calcium hydroxide and MTA: Histomorphometric study in rats. Archives of oral biology, 60(9), 1254-1262.
Floratos, S. G., & Kratchman, S. I. (2012). Surgical management of vertical root fractures for posterior teeth: report of four cases. Journal of endodontics, 38(4), 550-555.
Ford, T. R. P., Torabinejad, M., McKendry, D. J., Hong, C.-U., & Kariyawasam, S. P. (1995). Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 79(6), 756-763.
Fridland, M., & Rosado, R. (2005). MTA solubility: a long term study. J Endod, 31(5), 376-379.
Fuks A. Current concepts in vital primary pulp therapy. Eur J Paediatr Dent 2002; 3: 115 – 120
Fuks, A. B. (2008). Vital pulp therapy with new materials for primary teeth: new directions and treatment perspectives. J Endod, 34(7 Suppl), S18-24. doi:10.1016/j.joen.2008.02.031
Fuks A, Papagiannoulis L, Duggal M. Pulpotomy in primary teeth: Review of the literature according to standardized assessment criteria. Eur Arch Paediatr Dent 2006; 1(2): 64-72
Gandolfi, M., & Prati, C. (2010). MTA and F‐doped MTA cements used as sealers with warm gutta‐percha. Long‐term study of sealing ability. International endodontic journal, 43(10), 889-901.
Gandolfi, M., Taddei, P., Siboni, F., Modena, E., Ginebra, M., & Prati, C. (2011). Fluoride‐containing nanoporous calcium‐silicate MTA cements for endodontics and oral surgery: early fluorapatite formation in a phosphate‐containing solution. International endodontic journal, 44(10), 938-949.
Gandolfi, M. G., Ciapetti, G., Perut, F., Taddei, P., Modena, E., Rossi, P. L., & Prati, C. (2009). Biomimetic calcium-silicate cements aged in simulated body solutions. Osteoblast response and analyses of apatite coating. Journal of Applied Biomaterials and Biomechanics, 7(3), 160-170.
Gandolfi, M. G., Iacono, F., Agee, K., Siboni, F., Tay, F., Pashley, D. H., & Prati, C. (2009). Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 108(6), e39-e45.
Gandolfi, M. G., Siboni, F., Botero, T., Bossù, M., Riccitiello, F., & Prati, C. (2015). Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. Journal of applied biomaterials & functional materials, 13(1), 43-60.
Gandolfi, M. G., Taddei, P., Siboni, F., Modena, E., Ciapetti, G., & Prati, C. (2011). Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical–physical properties, bioactivity and biological behavior. Dental Materials, 27(7), e134-e157.
Gandolfi, M. G., Taddei, P., Tinti, A., Dorigo, E. D. S., & Prati, C. (2011). Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Materials Science and Engineering: C, 31(7), 1412-1422.
Garrocho-Rangel, A., Quintana-Guevara, K., Vazquez-Viera, R., Arvizu-Rivera, J. M., Flores-Reyes, H., Escobar-Garcia, D. M., & Pozos-Guillen, A. (2017). Bioactive Tricalcium Silicate-based Dentin Substitute as an Indirect Pulp Capping Material for Primary Teeth: A 12-month Follow-up. Pediatr Dent, 39(5), 377-382.
Giacomino C, Wealleans J, Kuhn N, Diogenes A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. Journal of Endodontics 2019; 45(1): 51-56.
Glass, R. L., & Zander, H. A. (1949). Pulp healing. J Dent Res, 28(2), 97-107. doi:10.1177/00220345490280021101
Gomes de Oliveira N, Rodrigues de Souza Araujo P, Torreão da Silveira, Sobrai A, Carvalho M. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur J Dent 2018; 12(2): 317-326
Grossman L. Obturation of root canal. In: Grossman L, ed. Endodontic Practice. 10th ed. Philadelphia, PA: Lea & Febiger; 1982:297
Güzeler, I., Uysal, S., & Cehreli, Z. C. (2010). Management of trauma‐induced inflammatory root resorption using mineral trioxide aggregate obturation: two‐year follow up. Dental Traumatology, 26(6), 501-504.
Hadrossek, P. H., & Dammaschke, T. (2014). New treatment option for an incomplete vertical root fracture–a preliminary case report. Head & face medicine, 10(1), 9.
Hashem, D., Mannocci, F., Patel, S., Manoharan, A., Brown, J. E., Watson, T. F., & Banerjee, A. (2015). Clinical and radiographic assessment of the efficacy of calcium silicate indirect pulp capping: a randomized controlled clinical trial. J Dent Res, 94(4), 562-568. doi:10.1177/0022034515571415
Hebling, J., Giro, E. M. A., & de Souza Costa, C. A. (1999). Biocompatibility of an adhesive system applied to exposed human dental pulp. Journal of endodontics, 25(10), 676-682.
Hilton, T. J., Ferracane, J. L., & Mancl, L. (2013). Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res, 92(7 Suppl), 16s-22s. doi:10.1177/0022034513484336
Holland, R., Bisco Ferreira, L., de Souza, V., Otoboni Filho, J. A., Murata, S. S., & Dezan, E., Jr. (2007). Reaction of the lateral periodontium of dogs’ teeth to contaminated and noncontaminated perforations filled with mineral trioxide aggregate. J Endod, 33(10), 1192-1197. doi:10.1016/j.joen.2007.07.013
Hörsted-Bindslev, P., Vilkinis, V., & Sidlauskas, A. (2003). Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 96(5), 591-600.
Huth K, Hajek-Al-Khatar N, Wolf P, Ilie N, Hickel R, Paschos E. Long-term effectiveness of four pulpotomy techniques: 3-year randomised controlled trial. Clin Oral Investig. 2012;16(4):1243–50
Iacono, F., Gandolfi, M. G., Huffman, B., Sword, J., Agee, K., Siboni, F., . . . Pashley, D. (2010). Push-out strength of modified Portland cements and resins. American journal of dentistry, 23(1), 43.
Jaber, L., Mascres, C., & Donohue, W. B. (1991). Electron microscope characteristics of dentin repair after hydroxylapatite direct pulp capping in rats. Journal of oral pathology & medicine, 20(10), 502-508.
Javed, F., Kellesarian, S. V., Abduljabbar, T., Gholamiazizi, E., Feng, C., Aldosary, K., . . . Romanos, G. E. (2017). Role of laser irradiation in direct pulp capping procedures: a systematic review and meta-analysis. Lasers Med Sci, 32(2), 439-448. doi:10.1007/s10103-016-2077-6
Katsamakis, S., Slot, D. E., Van der Sluis, L. W., & Van der Weijden, F. (2013). Histological responses of the periodontium to MTA: a systematic review. Journal of Clinical Periodontology, 40(4), 334-344.
Kitasako, Y., Inokoshi, S., & Tagami, J. (1999). Effects of direct resin pulp capping techniques on short-term response of mechanically exposed pulps. Journal of Dentistry, 27(4), 257-263.
Koubi, G., Colon, P., Franquin, J.-C., Hartmann, A., Richard, G., Faure, M.-O., & Lambert, G. (2013). Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—a prospective study. Clin Oral Investig, 17(1), 243-249.
Lee, S. J., Monsef, M., & Torabinejad, M. (1993). Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod, 19(11), 541-544. doi:10.1016/s0099-2399(06)81282-3
Luketić, S. F., Malčić, A., Jukić, S., Anić, I., Šegović, S., & Kalenić, S. (2008). Coronal microleakage of two root-end filling materials using a polymicrobial marker. Journal of endodontics, 34(2), 201-203.
Main, C., Mirzayan, N., Shabahang, S., & Torabinejad, M. (2004). Repair of root perforations using mineral trioxide aggregate: a long-term study. Journal of endodontics, 30(2), 80-83.
Marão, H. F., Panzarini, S. R., Aranega, A. M., Sonoda, C. K., Poi, W. R., Esteves, J. C., & Silva, P. I. S. (2012). Periapical tissue reactions to calcium hydroxide and MTA after external root resorption as a sequela of delayed tooth replantation. Dental Traumatology, 28(4), 306-313.
McHugh, C. P., Zhang, P., Michalek, S., & Eleazer, P. D. (2004). pH required to kill Enterococcus faecalis in vitro. Journal of endodontics, 30(4), 218-219.
Mejare I, Cvek M. Partial pulpotomy in young permanent teeth with deep carious lesions. Endod Dent Traumatol 1993; 9: 238-242
Nadin G, Goel BR, Yeung CA, Glenny AM. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev 2003; 1:CD0033220
Nair, P. N., Duncan, H. F., Pitt Ford, T. R., & Luder, H. U. (2008). Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J, 41(2), 128-150. doi:10.1111/j.1365-2591.2007.01329.x
Nekoofar, M., Adusei, G., Sheykhrezae, M., Hayes, S., Bryant, S., & Dummer, P. (2007). The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. International endodontic journal, 40(6), 453-461.
Nowicka, A., Łagocka, R., Lipski, M., Parafiniuk, M., Grocholewicz, K., Sobolewska, E., . . . Buczkowska-Radlińska, J. (2016). Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. BioMed research international, 2016.
Olsson, H., Petersson, K., & Rohlin, M. (2006). Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J, 39(6), 429-442. doi:10.1111/j.1365-2591.2006.01116.x
Oynick, J., & Oynick, T. (1985). Treatment of endodontic perforations. J Endod, 11(4), 191-192. doi:10.1016/s0099-2399(85)80146-1
Özgur B, Uysal S, Güngoör H. Partial Pulpotomy in Immature Permanent Molars After Carious Exposures Using Different Hemorrhage Control and Capping Materials. Pediatr Dent 2017; 39(5): 364-370.
Parirokh, M., & Torabinejad, M. (2010a). Mineral trioxide aggregate: a comprehensive literature review–Part I: chemical, physical, and antibacterial properties. J Endod, 36(1), 16-27. doi:10.1016/j.joen.2009.09.006
Parirokh, M., & Torabinejad, M. (2010b). Mineral trioxide aggregate: a comprehensive literature review–Part III: Clinical applications, drawbacks, and mechanism of action. J Endod, 36(3), 400-413. doi:10.1016/j.joen.2009.09.009
Parthasarathy, A., Kamat, S. B., Kamat, M., & Kidiyoor, K. H. (2016). Histological response of human pulps capped with calcium hydroxide and a self-etch adhesive containing an antibacterial component. Journal of conservative dentistry: JCD, 19(3), 274.
Paula, A. B., Laranjo, M., Marto, C. M., Paulo, S., Abrantes, A. M., Casalta-Lopes, J., . . . Carrilho, E. (2018). Direct Pulp Capping: What is the Most Effective Therapy?-Systematic Review and Meta-Analysis. J Evid Based Dent Pract, 18(4), 298-314. doi:10.1016/j.jebdp.2018.02.002
Petrou, M. A., Alhamoui, F. A., Welk, A., Altarabulsi, M. B., Alkilzy, M., & C, H. S. (2014). A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin Oral Investig, 18(5), 1383-1389. doi:10.1007/s00784-013-1107-z
Prati, C., & Gandolfi, M. G. (2015). Calcium silicate bioactive cements: biological perspectives and clinical applications. Dental Materials, 31(4), 351-370.
Primus, C. M., Tay, F. R., & Niu, L.-n. (2019). Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomaterialia, 96, 35-54. doi:https://doi.org/10.1016/j.actbio.2019.05.050
Rafter, M. (2005). Apexification: a review. Dental Traumatology, 21(1), 1-8. doi:10.1111/j.1600-9657.2004.00284.x
Rajasekharan, S., Martens, L., Cauwels, R., & Anthonappa, R. P. (2018). Biodentine™ material characteristics and clinical applications: a 3 year literature review and update. European Archives of Paediatric Dentistry, 19(1), 1-22.
Redig D. A comparison and evaluation of two formocresol pulpotomy techniques utilizing “Buckley’s” formocresol. ASDC J Dent Child 1968;35:22-30.
Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater 2008; 24: 149 –164.
Saatchi, M., Shadmehr, E., Talebi, S. M., & Nazeri, M. (2013). A prospective clinical study on blood mercury levels following endodontic root-end surgery with amalgam. Iranian endodontic journal, 8(3), 85.
Saunders, W. P. (2008). A prospective clinical study of periradicular surgery using mineral trioxide aggregate as a root-end filling. Journal of endodontics, 34(6), 660-665.
Schmitt, D., Lee, J., & Bogen, G. (2001). Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent, 23(4), 326-330.
Schuurs, A., Gruythuysen, R., & Wesselink, P. (2000). Pulp capping with adhesive resin‐based composite vs. calcium hydroxide: a review. Dental Traumatology: Review article, 16(6), 240-250.
Seo D-G, Lee D, Kim Y-M, Song D, & Kim S-Y.Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials (Basel) 2019, 12(15). doi:10.3390/ma12152482
Sharifi, R., Parirokh, M., Satvati, S. A. R., & Torabinejad, M. (2014). Treatment of inflammatory root resorption using mineral trioxide aggregate: A case report. Dental Hypotheses, 5(4), 172.
Siew, K., Lee, A. H. C., & Cheung, G. S. P. (2015). Treatment Outcome of Repaired Root Perforation: A Systematic Review and Meta-analysis. Journal of endodontics, 41(11), 1795-1804. doi:https://doi.org/10.1016/j.joen.2015.07.007
Smaïl-Faugeron V, Glenny AM, Courson F, Durieux P, Muller-Bolla M, Fron Chabouis H. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev 2018; 5: CD003220
Song, M., & Kim, E. (2012). A prospective randomized controlled study of mineral trioxide aggregate and super ethoxy-benzoic acid as root-end filling materials in endodontic microsurgery. J Endod, 38(7), 875-879. doi:10.1016/j.joen.2012.04.008
Sonmez D, Duruturk L. Ca(OH)2 pulpotomy in primary teeth. Part I: internal resorption as a complication following pulpotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106:94–98.
Stanley, H. R. (1989). Pulp capping: conserving the dental pulp–can it be done? Is it worth it? Oral Surg Oral Med Oral Pathol, 68(5), 628-639. doi:10.1016/0030-4220(89)90252-1
Stringhini Junior E, Vitcel ME, Oliveira LB. Evidence of pulpotomy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol. European Archives of Paediatric Dentistry 2015; 16(4):303–12.
Suzuki, M., Katsumi, A., Watanabe, R., Shirono, M., & Katoh, Y. (2005). Effects of an Experimentally Developed Adhesive Resin System and CO~ 2 Laser Irradiation on Direct Pulp Capping. OPERATIVE DENTISTRY-UNIVERSITY OF WASHINGTON-, 30(6), 702.
Sweet C. Procedure for treatment of exposed and pulpless deciduous teeth. J Am Dent Assoc 1930;17:1150-1153.
Taddei, P., Modena, E., Tinti, A., Siboni, F., Prati, C., & Gandolfi, M. G. (2014). Effect of the fluoride content on the bioactivity of calcium silicate-based endodontic cements. Ceramics International, 40(3), 4095-4107.
Taschieri, S., Tamse, A., Del Fabbro, M., Rosano, G., & Tsesis, I. (2010). A new surgical technique for preservation of endodontically treated teeth with coronally located vertical root fractures: a prospective case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 110(6), e45-52. doi:10.1016/j.tripleo.2010.07.014
von Arx, T., Hänni, S., & Jensen, S. S. (2014). 5-year results comparing mineral trioxide aggregate and adhesive resin composite for root-end sealing in apical surgery. Journal of endodontics, 40(8), 1077-1081.
von Arx, T., Jensen, S. S., & Hanni, S. (2007). Clinical and radiographic assessment of various predictors for healing outcome 1 year after periapical surgery. J Endod, 33(2), 123-128. doi:10.1016/j.joen.2006.10.001
von Arx, T., Jensen, S. S., Hanni, S., & Friedman, S. (2012). Five-year longitudinal assessment of the prognosis of apical microsurgery. J Endod, 38(5), 570-579. doi:10.1016/j.joen.2012.02.002
Watts, J. D., Holt, D. M., Beeson, T. J., Kirkpatrick, T. C., & Rutledge, R. E. (2007). Effects of pH and mixing agents on the temporal setting of tooth-colored and gray mineral trioxide aggregate. Journal of endodontics, 33(8), 970-973.
Weldon, J. K., Jr., Pashley, D. H., Loushine, R. J., Weller, R. N., & Kimbrough, W. F. (2002). Sealing ability of mineral trioxide aggregate and super-EBA when used as furcation repair materials: a longitudinal study. J Endod, 28(6), 467-470.
Zander H. Reaction of the pulp to calcium hydroxide. J Dent Res 1939; 18:373-379.